Ten Steps to Commercializing a Great Idea
1) I've got a great, revolutionary idea. How do I pursue it?
 That depends on the impact if your idea could be turned into something practical. Some examples of great questions that have led to government grants are: Might tissue lost to trauma and disease be restored? Can tooth decay be identified and treated in its earliest stages? Could populations be screened for exposure to dangerous pathogens by rapid analysis of spit? Great ideas commonly spring from frustration with the limits of the state-of-the-art, or the recognition of an urgent knowledge gap by practitioners in the field. Often this leads to an insight into a possible solution that needs to be tested, validated, and developed. One possible route is to start a business to develop the concept into a marketable product. Excellent guidance for determining if this path is right for you can be found at the "Startup Basics" section of the Small Business Administration website.
That depends on the impact if your idea could be turned into something practical. Some examples of great questions that have led to government grants are: Might tissue lost to trauma and disease be restored? Can tooth decay be identified and treated in its earliest stages? Could populations be screened for exposure to dangerous pathogens by rapid analysis of spit? Great ideas commonly spring from frustration with the limits of the state-of-the-art, or the recognition of an urgent knowledge gap by practitioners in the field. Often this leads to an insight into a possible solution that needs to be tested, validated, and developed. One possible route is to start a business to develop the concept into a marketable product. Excellent guidance for determining if this path is right for you can be found at the "Startup Basics" section of the Small Business Administration website.
Pursuing your inspiration will invariably involve time and money, and it is never too early to explore the great diversity of funding mechanisms available within the U.S. federal government that might help realize the dream. At the National Institutes of Health, a great place to start is by surveying the mission statements of the various institutes and centers (ICs) to see if your concept is consistent with their interests. Often the ICs will list their funding priorities prominently on their web sites. See NIDCR's funding opportunity announcements. Alternatively, contact the NIDCR Small Business Coordinator who can help put you in touch with the proper Program Director with a portfolio that fits with your idea, and who can mentor you through the early stages of applying for funding.
2) What other solutions are out there? Do they represent the competition, or potential collaborators?
NIH provides an excellent resource in the Research Portfolio Online Reporting Tools (RePORT). RePORT contains the abstracts of all NIH-funded projects, and is searchable under key words, funding mechanisms and sources, organization, principal investigator, etc. Searches will provide a list of existing NIH investments that generally relate to your idea, an indication of the state of the science, and the names of potential collaborators and/or competitors. Additionally, PubMed is the largest collection of abstracts of published scientific articles in the world, and can provide a broad sense of where the research stands in areas that relate to your discovery. Again, the search will provide the names of others active in the field.
Your state or local government may also provide a center or office to help small business owners and potential owners identify possible partners and/or business opportunities. You can get some valuable information about the possible resources in your state from the Manufacturing Extension Partnership (MEP) website External Web Site Policy at the National Institutes of Standards and Technology (NIST).
Remember, inspiration is not invention! Do your homework before approaching others and they will be easier to engage. Define your vision first. This involves critically assessing the current state-of-the-art and quantifying the difference that your idea will make if fully deployed. Which related technologies are now commercially available? Which are under development? Will my contribution make incremental improvements, or change the whole field? Such "market research" is NOT about sales projections, but all about describing the appropriate goal and the requirements to get there.
3) Do I need a partner?
 Probably. Today's technologies are generally too sophisticated to be fully deployed by any single organization. Your early market research should identify possible collaborators to help develop and hone your ideas. You must be credible when approaching potential partners, so be prepared. Visit web sites to get ideas for placement of your technology within your potential partner's organization, and position your ideas to complement their development plans. Get their attention first, and make them really want to see what you can offer. You should engage such development partners as early as practicable. A serious commercialization partner will be willing to share in prosecuting the patent(s) in addition to adding technical and business expertise to the team.
Probably. Today's technologies are generally too sophisticated to be fully deployed by any single organization. Your early market research should identify possible collaborators to help develop and hone your ideas. You must be credible when approaching potential partners, so be prepared. Visit web sites to get ideas for placement of your technology within your potential partner's organization, and position your ideas to complement their development plans. Get their attention first, and make them really want to see what you can offer. You should engage such development partners as early as practicable. A serious commercialization partner will be willing to share in prosecuting the patent(s) in addition to adding technical and business expertise to the team.
Once you establish a mutual interest, you need to sign agreements to keep your discussions confidential to the principals. Model agreements are readily available and many universities provide forms through their technology transfer offices. Know the "ins and outs" of these often misunderstood documents! Read a good discussion on the NIH's view on a diversity of issues in technology transfer. Early execution of such "Non Disclosure Agreements" demonstrates care and attention to detail—preparation, not paranoia.
4) Should I patent my idea?
Yes, in the majority of cases. Any idea with commercial potential requires some safeguards. Even if you decide that your innovative idea should be generally accessible by all, you must provide the coverage that a patent offers on how the possible applications will be realized. Visit NIH's technology transfer web site for some helpful hints.
Once you begin to invest significant resources of your own and your early associates, you must have some guarantees that your product can be distributed and marketed according to your vision. Permission to use your patent can be assigned under a wide umbrella from exclusive (i.e. one licensee) to freely and publicly available. You get to decide. But without the right to exercise the control that comes with owning the intellectual property—the patent—no one will develop your invention to its full potential. Most startup companies are valued almost entirely on the estimated worth of an exclusive market that comes with the founding patents for the technology. Information about patent protection is available from the United States Patent and Trademark Office (USPTO).
While the concept and process of applying for a patent is straightforward in principal, the stakes for highly profitable inventions can be very high. That is why most companies (even startups) engage patent attorneys even before CEOs! Many law firms often have a graduated fee structure that allows companies to utilize basic initial services cheaply and move to a higher level of service when the need arises. A little research can payoff here. Tap into local resources both geographically (e.g. your local Chamber of Commerce, venture capital discussion group, or state technology development resources) and among your industry friends to get a short list of attorneys who are knowledgeable and successful in your technical area. Unfortunately, the rising costs of pursuing a patent force some inventors to abandon the protection of every discovery. But that decision should be made only after a careful evaluation that is part of the early preparation of the patent paperwork. In lieu of a patent, you may decide to hold the idea as a "trade secret", and this pathway, too, requires limitations on discussions. There is no substitute for good legal advice and the rigor to follow it, even at this early stage.
5) How do I move from promising research to serious development?
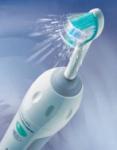
A power toothbrush (shown here) that incorporates ultrasound has been developed by Second Act Partners, Inc. It rapidly and safely removes plaque at levels well beyond the capabilities of existing power toothbrushes.
There are many pathways to success. Just as inspiration is not invention, invention is not innovation! A vague notion of a better world needs to be laid out in discrete steps that lead logically from one to the other. It cannot be stressed enough that while pursing the development of your technology may be your dream, that dream needs to be firmly rooted in a practical framework. If you are a university-based researcher, you can start a company or establish a relationship with like-minded corporate scientists and/or developers. If you are based in industry, you may need to convince the development strategists within your company to re-allocate resources to develop your idea, as well as recruit academic collaborators to the effort. From your earliest discussions, you may recognize that capital from outside of your current organization will most likely be needed. There are several paths that may be considered (some simultaneously) to move forward with your ideas.
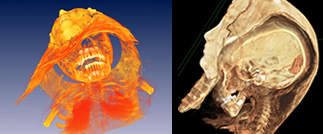
 Xoran Technologies, Inc has developed a dentomaxillofacial cone-beam CT scanner. This new technology allows greater resolution for both soft and hard tissues imaging, and is safer because its focused beam produces less extraneous X-irradiation.
Xoran Technologies, Inc has developed a dentomaxillofacial cone-beam CT scanner. This new technology allows greater resolution for both soft and hard tissues imaging, and is safer because its focused beam produces less extraneous X-irradiation.
It is worth repeating that the federal government is a great place to seek funding for further development of a discovery. Small businesses are eligible for Small Business Innovation Research (SBIR) or Small Business Technology Transfer Research (STTR) grants. Over $3B is distributed annually through the various federal agencies that fund research. See more about NIH's SBIR and STTR programs. Other agencies have similar web sites. Bear in mind that biomedical research has a broad reach, and may be of interest to multiple federal funders. For example, chip-based diagnostic technologies have been funded through NIH, as well as the National Science Foundation (NSF), the Defense Advance Research Projects Agency (DARPA) in the Department of Defense, Homeland Security Advanced Research Projects Agency (HSARPA), the Advanced Technology Program (ATP) at the Department of Commerce (DOC), the "Genomes to Life" initiative at the Department of Energy (DOE), the National Aeronautics and Space Administration (NASA), and others.
While government grants are a good way to advance specific technologies, you must also attend to establishing a solid business that will eventually market your product or service. You may consider and compete for everything from a convertible debt from a small investor to a venture capital firm's large commitment. Bear in mind that the greater the support from such private sources, the more your must surrender control of the company. Essentials of a good business plan for growing companies can be found at the Small Business Administration's site. External Web Site Policy This effort takes persistence, as most entrepreneurs state that it may take one hundred contacts to lead to a single investment. The key to all of these private placements is networking and constantly revisiting and refining the corporate message. State assistance programs and the local Manufacturing Extension Partnership (MEP) may be helpful here as well.
6) So, NIDCR might support my research and development, but how do I determine where to send my application, or which aspects are of most interest?
 Here again, the web is a great resource. By searching the various home pages, you will discover that the NIH mission is to make "important medical discoveries that improve health and save lives". The NIDCR embraces that overall vision with its focus squarely on orofacial health. The Institute's goals are in great measure fulfilled by providing support for experimental science and technology development via awarding research grants and contracts. So, a grant application focused on dental or craniofacial research challenges will be directed to NIDCR as the potential funding source. In "NIH-speak", the cognoscente Institute or Center (IC) would be NIDCR (often abbreviated as DE). Applications are submitted by institutions (e.g. universities, non-profit organizations, companies) on behalf of the Principal Investigator (PI), who is the person primarily responsible for guiding the scientific and technical aspects of the research.
Here again, the web is a great resource. By searching the various home pages, you will discover that the NIH mission is to make "important medical discoveries that improve health and save lives". The NIDCR embraces that overall vision with its focus squarely on orofacial health. The Institute's goals are in great measure fulfilled by providing support for experimental science and technology development via awarding research grants and contracts. So, a grant application focused on dental or craniofacial research challenges will be directed to NIDCR as the potential funding source. In "NIH-speak", the cognoscente Institute or Center (IC) would be NIDCR (often abbreviated as DE). Applications are submitted by institutions (e.g. universities, non-profit organizations, companies) on behalf of the Principal Investigator (PI), who is the person primarily responsible for guiding the scientific and technical aspects of the research.
For-profit entities are eligible for many of the different types of awards, but the small business grants are the primary mechanism by which corporate science gets assistance. The range of available opportunities and the grant application process is described in great detail at the NIH's SBIR/STTR web site. The explanations are very thorough, but it is helpful to keep certain cultural aspects of the organization in mind in approaching grant writing. So, remember: It's all about the science! A well-crafted and presented research plan is a critical part of the presentation. You must be able to articulate your program in a few richly referenced and logically related specific aims that move from the current state-of-the-art to a well-defined end point. These aims must be rooted in fact, and carefully elaborated in a fully referenced and defended research plan. At NIH, experience counts, so adding competent collaborators with track records in the field will improve your chances of success.
Consider and define roles with care—the principal investigator is not always the inventor. Do not undertake a collaboration when a subcontract or consulting arrangement will suffice. The key is to bring on critical expertise at the right level of involvement to move the research along efficiently. Adding experts with name recognition in the field but no real involvement in the project will not make the application more competitive.
It is worth noting that small businesses can participate at many levels in NIH awards. In addition to the SBIR and STTR programs, most other granting mechanisms are open to corporate applicants, and companies are frequent subcontractors or consultants on NIH grants and contracts. It is wise to look to many sources to support your technology development goals.
7) How competitive is the granting process? How does the NIH review applications and decide which to fund?
The competition is stiff, and the NIH review is meticulous. Currently, less than 20% of the applications received across all of the many NIH granting mechanisms are funded. You must combine an important and timely idea with a carefully crafted and well documented research plan from a highly skilled team into a presentation that appeals to the reviewers and also addresses a key concern of the funding institute or center. However, the payoff is a solid base of support to pursue the development of the technology. The government does not require something tangible in return for its investment. NIH's only interest is in supporting a rigorous, science-based effort that has the best chance of solving the problem at hand.
 Review of grant applications requires the focused input of several dozen professionals from many disciplines. NIH receives almost 80,000 grant or contract applications annually, which are first evaluated for technical merit by teams of peer reviewers informally called "study sections", or more properly Integrated Review Groups (IRGs). The significance, approach, innovation, investigator, and environment are assessed, and a priority score assigned by the review committee. Applications are further assessed for their alignment to the goals of the various funding components (Institutes or Centers) that together comprise the NIH. Applications recommended for funding are further evaluated against administrative guidelines and procedures, and adherence with all eligibility requirements. A very good overview of the process with deadlines, timelines and grant cycles can be found on the Office of Extramural Research web site. Also see more information about peer review. It is important to realize that such a thorough evaluation is deliberative, and it currently takes at least nine months between proposal submission and the initiation of any funding.
Review of grant applications requires the focused input of several dozen professionals from many disciplines. NIH receives almost 80,000 grant or contract applications annually, which are first evaluated for technical merit by teams of peer reviewers informally called "study sections", or more properly Integrated Review Groups (IRGs). The significance, approach, innovation, investigator, and environment are assessed, and a priority score assigned by the review committee. Applications are further assessed for their alignment to the goals of the various funding components (Institutes or Centers) that together comprise the NIH. Applications recommended for funding are further evaluated against administrative guidelines and procedures, and adherence with all eligibility requirements. A very good overview of the process with deadlines, timelines and grant cycles can be found on the Office of Extramural Research web site. Also see more information about peer review. It is important to realize that such a thorough evaluation is deliberative, and it currently takes at least nine months between proposal submission and the initiation of any funding.
Assuring appropriate review of the application is key, especially if your research addresses an emerging challenge, or integrates more than one field of investigation. No one knows the technology better than you, the proposer. You can help in assigning the merit review to the committee with the best expertise. The Center for Scientific Review (CSR) serves as the central receipt and distribution point for all research grant applications submitted to the NIH.
8) What should I do as the research process moves forward?
 Keep looking ahead to the next big hurdle in the development of your technology. An award from the NIH provides the necessary funds to support the research, and it also shows that the experimental plan was highly rated by the peer reviewers. Such a technical validation along with a source of undiluted capital (i.e. a cash infusion in which the investor takes no specific ownership interest in the performing organization) improves a young company's outlook immeasurably. Companies often find that there is a positive aura from such early stage support that makes the project appealing to other investors. So, once your NIH grant is awarded, you might actively advertise that support with press releases and poster presentations at industry meetings.
Keep looking ahead to the next big hurdle in the development of your technology. An award from the NIH provides the necessary funds to support the research, and it also shows that the experimental plan was highly rated by the peer reviewers. Such a technical validation along with a source of undiluted capital (i.e. a cash infusion in which the investor takes no specific ownership interest in the performing organization) improves a young company's outlook immeasurably. Companies often find that there is a positive aura from such early stage support that makes the project appealing to other investors. So, once your NIH grant is awarded, you might actively advertise that support with press releases and poster presentations at industry meetings.
It is also important to plan early for the later stages of development of the technology. Most medical products require regulatory approval by the US Food and Drug Administration (FDA). This process formalizes a series of safety and efficacy studies that generally fall into three phases before market approval and increasingly, a fourth follow up study once the product is being used (see the FDA website External Web Site Policy for an overview and a more detailed analysis for new drugs). There are three main centers at the FDA which oversee such approvals, with some "combination" products falling under joint jurisdiction of two or three of these groups. The purview and operating procedures of each center are summarized on their respective web sites: the Center for Drug Evaluation and Research (CDER) External Web Site Policy, the Center for Biologics Evaluation and Research (CBER) External Web Site Policy, and the Center for Devices and Radiological Health (CDRH) External Web Site Policy. Pursuing the long and complex approval process is a necessary step in securing the latest technologies for improving public health, and early education in this arena allows a competitive advantage in designing experimental protocols to best meet FDA requirements.
Medical and dental therapeutics are becoming increasingly complex, and the intricacy of paying for new treatments is also growing rapidly. Successful marketing requires a very sophisticated effort from a knowledgeable team. For example, FDA approval is not enough to insure access to your product. While patients are the ultimate beneficiaries, medical professionals actually order the products, and third parties (e.g. Health Maintenance Organizations (HMOs), Preferred Provider Associations (PPAs), and insurers) foot most of the bill. Savvy biotechnology, pharmaceutical, and medical device companies learn the "ins and outs" of "reimbursement" early. The Centers for Medicare and Medicaid Services (CMS) sets requirements for federal reimbursement programs and these guidelines become industry standards.
9) How do I network to commercialize my new product?
Shrewdly. Astute project directors should look for late stage investors and development partners as the feasibility of a proposed new product or service is established, and the work shifts toward clinical validation.
Many larger biotechnology companies and pharmaceutical companies regularly scan the literature and presentations at scientific meetings for emerging opportunities. You need to be at such meetings, with high profile presentations. As your research matures your technology may become increasingly noticed, and the range of investment opportunities expanded. This is where true partnerships emerge and licensing, joint ventures, and possibly mergers/acquisitions begin. Take advantage of any opportunity which focuses on showcasing the newest technologies in your preferred market. Local venture capital forums attract local investors, which can be key in the early stages of development. Large meetings may be ideal for generating excitement among development and marketing partners. Potential investors will want to see how you and your product attract attention amid the distracting competition.
Maintaining portfolio balance becomes an increasing challenge. Constant attention to the business plan and good communication between the technical team and the business strategists is a must to keep the company's research and development activities in line with the mission and vision.
10) Your idea is a success. What next?
Marketing a successful product is a major milestone in the life of any company. However, long term success requires multiple marketable offerings. Growing companies in the medical therapeutics arena often develop several types of products around related themes.
Once a firm has positive cash flow, doors begin to open. In today's competitive environment, track record matters. A primary goal for a start-up company should be a rapid path to sales on multiple fronts. Related products should be rolled out as soon as possible to diversify the revenue streams, even though income may initially be small from each new offering. Most investors would prefer to expand, rather than open, a new market.
As more and more people develop and use the technology, other applications come to light. Further development requires more experimentation, and the cycle can begin again with further federal grants and private investments. The company may elect to spin off another entity to concentrate development efforts on these new possibilities. Converting a competitor to a collaborator via licensing and co-development agreements is also a possibility. Paths diverge significantly once such opportunities open up. Yet the process always comes full circle. Innovation is a constant challenge, and vital to sustaining competitive advantage long term.