Although vaccines and treatments eased pandemic restrictions, access to accurate and affordable tests remains a persistent challenge. Anavasi Diagnostics, a medical technology company based in Redmond, Washington, is helping overcome this hurdle through the development of a portable, easy-to-use assay that can detect the SARS-CoV-2 virus in under 20 minutes.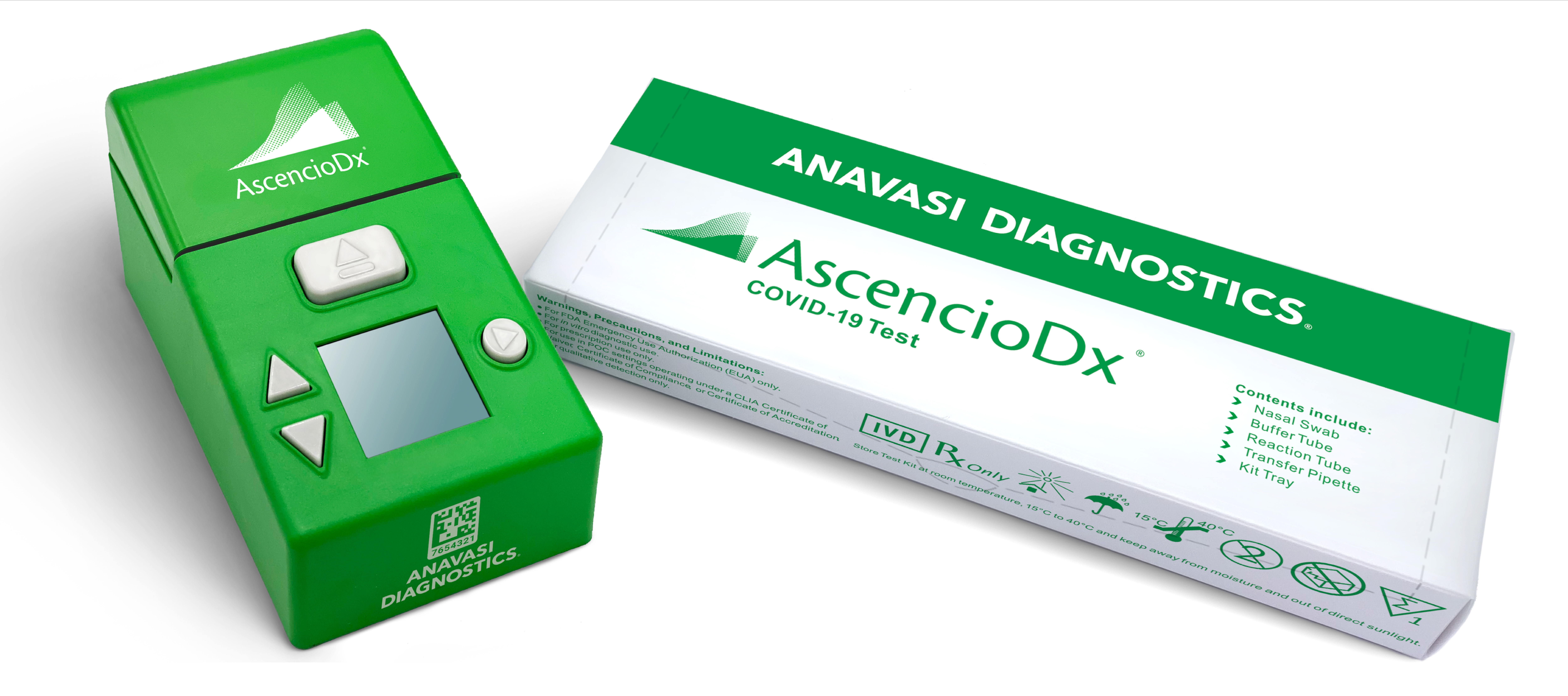
In January 2020, Barry Lutz, co-founder and chief scientific officer of Anavasi Diagnostics and bioengineer at the University of Washington in Seattle, and his graduate student team, including Robert Atkinson (a PhD candidate under Lutz) began developing an assay that detects several regions of the SARS-CoV-2 virus. In August 2020, Lutz and Atkinson received funding from The Center for Washington Entrepreneurial Research Evaluation & Commercialization Hub (WE-REACH), a program supported in part by the National Institutes of Health (NIH), to assist in the development and commercialization of their assay. They brought on Minh Duong as a co-founder and began working with Steve Flaim, an Entrepreneur in Residence at NIH’s SEED Office. The team optimized their technology, navigated business logistics, and secured $14.9 million of funding in September 2021 through NIH’s Rapid Acceleration of Diagnostics (RADx) initiative, which aims to speed up the development, commercialization, and implementation of COVID-19 testing technologies.
One unique aspect of Anavasi’s AscencioDx COVID-19 Test is that is able to detect all variants of the virus identified to date because it looks at multiple locations in the genetic code as opposed to many other tests that only look at one region of the virus. The test is also inexpensive and easy to operate, and the assay is much faster than other tests. PCR tests require a central lab and at least eight hours, if not two to three days, for results. “In our clinical trial of the assay, positive results came back, on average, in 16.8 minutes,” says Nelson Patterson, president and CEO of Anavasi Diagnostics. A further advantage is that the AscencioDx COVID-19 Detector hardware is reusable for approximately 3,000 tests, making it more environmentally friendly than other single-use tests that dispose of plastic, chemistry, electronics and batteries after running each test.
We would not be where we are, were it not for RADx… NIH was indispensable, frankly, to our success.
In February 2023, Anavasi received Emergency Use Authorization from the Food and Drug Administration for the AscencioDx COVID-19 Test and Molecular Detector, making the testing devices available to medical professionals offering point-of-care services at urgent care and emergency care centers, and at senior living centers, skilled nursing facilities, assisted living facilities, and mobile testing sites for social events and large gatherings.
“We would not be where we are were it not for RADx. The supply chain and manufacturing support sustained us. The commercial expertise and regulatory resources we got were fantastic. They helped us understand the FDA process,” says Patterson. “NIH was indispensable, frankly, to our success.”
Now, Anavasi is looking for further ways to advance diagnostics by expanding use of their technology to other viral and bacterial diseases like flu, strep throat, and RSV. Doing so will help address global challenges with health inequity, according to Patterson. “Many people are dying because they don’t have good access to timely diagnostics,” he says. “But you can bring point-of-care molecular diagnostics to any neighborhood, particularly those that are the underserved. That is our mission, and we are fortunate that the NIH/RADx program has enabled it.”







