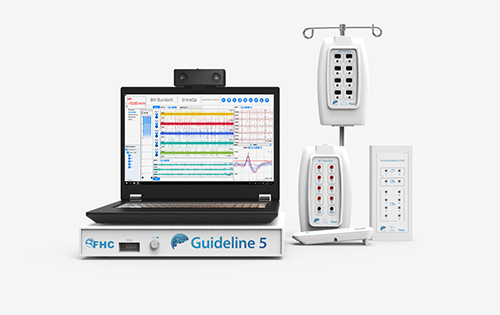
Treatments for movement disorders such as Parkinson’s disease, epilepsy, dystonia, and essential tremor range from physical and occupational therapy to medications to deep brain stimulation (DBS). FHC, founded by Fred and Jill Haer in 1970, is a leading DBS company based in Bowdoin, ME that develops the tools and instruments neurosurgeons use to treat movement disorders, primarily those that directly access and “microtarget” parts of the brain.
Haer credits FHC’s first NIH Small Business Innovative Research (SBIR) grant from the National Institute of Neurological Disorders and Stroke (NINDS), awarded in 1997, with helping the company make the leap from research and development to clinical application. It all began with FHC’s core product—a metal microelectrode with a sharp tip that, once inserted in the brain, records activity of nearby cells. Some 30-plus years ago scientists became interested in a microelectrode that could do more, and those scientists reached out to FHC. To determine what improvements could be made to the single microelectrode, FHC turned to SBIR grants. The result was a trimodal microelectrode system that enabled medical professionals to simultaneously record and stimulate brain cells, electronically control the device, and microposition the device in the brain.
The device manufacturer Medtronic expressed a growing interest in further advancing the trimodal microelectrode system for possible use for DBS. In the early 2000s, it made a small investment in FHC, and together, Medtronic and FHC forged a collaborative partnership where FHC focused on the research while Medtronic assisted in the clinical testing and regulatory approval process. Today, the majority of FHC products are used for DBS.
FHC develops the tools and instruments neurosurgeons use to treat movement disorders, primarily those that directly access and “microtarget” parts of the brain.
A participant in the 2010-2011 NIH Commercialization Accelerator Program (CAP), Haer recalls that it was very productive, noting that some companies skip or rush through the basic market analysis and information-gathering that is needed to build a strong company foundation.  The NIH CAP encourages businesses to do this during the project itself, making it a worthwhile program in which to be involved.
The NIH CAP encourages businesses to do this during the project itself, making it a worthwhile program in which to be involved.
Later, the National Cancer Institute and National Institute of Mental Health supported FHC with multiple SBIR and STTR (Small Business Technology Transfer) awards primarily for additional research and development of the DBS trimodal tool, for analyzing results of its use, or for tissue sampling.
FHC collaborates with scientists, physicians, technologists and medical device companies to research and develop instruments and tools that practitioners successfully use in a clinical setting. Haer notes that, “we’ve used the NIH SBIR vehicle when we had a project that we were interested in and to obtain support for that research. We see the NIH SBIR as a means by which we develop technologies for others, who in turn may go on to conduct additional product research or commercialization.”







