 The Centers for Disease Control and Prevention estimates the number of Americans with age-related macular degeneration (AMD)—the leading cause of vision loss in the U.S—will rise to almost three million in 2020. Not only does vision loss lead to a decrease in independence and increase in accidents, it burdens primary care givers and increases mental health issues. Until recently, there were no treatment options for the most common form of AMD.
The Centers for Disease Control and Prevention estimates the number of Americans with age-related macular degeneration (AMD)—the leading cause of vision loss in the U.S—will rise to almost three million in 2020. Not only does vision loss lead to a decrease in independence and increase in accidents, it burdens primary care givers and increases mental health issues. Until recently, there were no treatment options for the most common form of AMD.
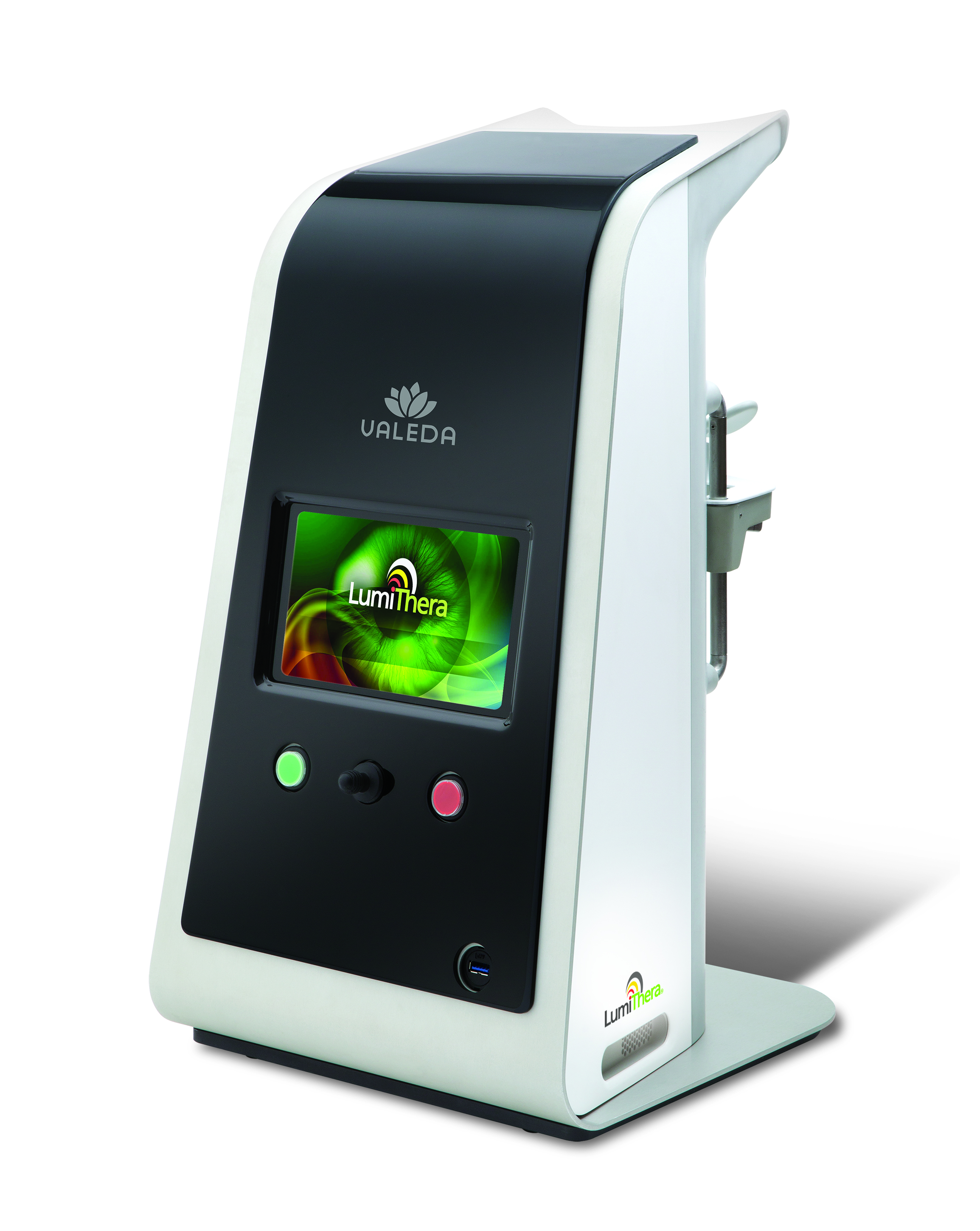
The lack of treatment options was one of the reasons the founders of LumiThera started a company in 2013. “The goal of the company was to create a noninvasive, safe treatment,” says Clark Tedford, LumiThera co-founder, president and CEO.
The LumiThera team developed a technique called photobiomodulation, which stimulates energy production and increased blood flow to cells in the eyes through light therapy. The treatment takes less than ten minutes, does not require pupil dilation, and can sometimes be performed through closed eyelids. “We're trying to strengthen the retinal tissue to make it healthier,” says Tedford.
In 2015 LumiThera was awarded two Phase I Small Business Innovative Research (SBIR) grants from the National Eye Institute (NEI), one to continue basic research and one to conduct a pilot study. The results from the study, called Light Sight I, showed that vision in people with dry AMD was improved after patients received light treatment.
The LumiThera team developed a technique called photobiomodulation, which stimulates energy production and increased blood flow to cells in the eyes through light therapy.
The company was awarded a Phase II SBIR grant from the NEI in 2019, which allowed them to launch Light Sight III clinical trials. The team is conducting the clinical trial in five countries, including in ten U.S. centers. Tedford says results from the study will help the company obtain FDA approval for their light treatment, which could be granted as early as 2022.
Tedford says support from the SBIR grants has given the company credibility through the peer review process, which has provided validation of their technology in and outside of the U.S.
Aside from AMD, LumiThera is working on treating other causes of blindness, like cataracts, glaucoma, and diabetic retinopathy, the latter of which is the leading cause of new cases of blindness in adults. LumiThera just submitted a Direct to Phase II SBIR grant in January 2019 to conduct a pilot study investigating light therapy as a treatment for diabetic retinopathy.
Tedford credits NIH support for helping LumiThera get traction and thinks the program is important for small business success. “There are some very good companies with great ideas that use the [SBIR grants] to get out of the gate. That early funding is very critical.”







