 When a layoff left Anne Marie Quinn out of a job in the mid-2000s, she decided she was ready to start her own business—Montana Molecular—in Bozeman, Montana. But she needed seed money to get started and venture capital was tough to acquire.
When a layoff left Anne Marie Quinn out of a job in the mid-2000s, she decided she was ready to start her own business—Montana Molecular—in Bozeman, Montana. But she needed seed money to get started and venture capital was tough to acquire.
Using state incubator funding, Quinn’s team successfully submitted a Small Business Technology Transfer (STTR) grant to the NIH in 2008, which allowed the company to develop fluorescent sensors to study neurological diseases in living cells. Since then, Montana Molecular has received more than four million dollars in STTR and Small Business Innovative Research (SBIR) funding from four NIH Institutes. The grants have helped the company develop a large library of products that researchers use to study many types of diseases, from substance use disorders to diabetes, as well as test the toxicity and effectiveness of new drugs in cells before they are tested in animals.
Earlier this year Montana Molecular applied its expertise in live-cell assay development to COVID-19 and developed two new products. The first is a sensor that measures replication of the novel coronavirus, SARS-CoV-2, and whether certain drugs can stop the process. The second is a pseudovirus, a template virus coated with SARS-CoV-2 markers that can gain entry into cells but doesn’t cause infection. The pseudovirus will allow researchers to study how SARS-CoV-2 enters cells—and how to prevent it—without requiring expensive biosecurity lab setups. Quinn says researchers have reported early success with the pseudovirus in lung cells.
[SBIR] funding was absolutely critical to getting us off the ground
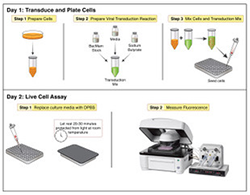 But switching focus had its risks. “This is probably the first time in the history of the company that we could use our sales revenue [to] support our own R and D,” says Quinn, who partially credits the SBIR program for supporting Montana Molecular’s ability to take that risk. “[SBIR] funding was absolutely critical to getting us off the ground.”
But switching focus had its risks. “This is probably the first time in the history of the company that we could use our sales revenue [to] support our own R and D,” says Quinn, who partially credits the SBIR program for supporting Montana Molecular’s ability to take that risk. “[SBIR] funding was absolutely critical to getting us off the ground.”
Today the company has commercialization partnerships and distributors in at least seven countries for products to be used by basic scientists in university labs and drug discovery teams at biotech and pharmaceutical companies. From 2018 to 2019 their profits grew more than 100 percent, and researchers have published more than 50 peer-reviewed articles with a connection to a Montana Molecular product. Montana Molecular currently employs nine staff members and three consultants. The company recently surpassed one million dollars in sales revenue.
 Quinn says product success and publication numbers aren’t the only benefits of SBIR support. An I-Corps grant from the National Institute of Neurological Disorders and Stroke provided professional development training for Kevin Harlan, a Montana Molecular early-career scientist interested in technology transfer. Harlan recently started his own biotechnology consulting company in Montana.
Quinn says product success and publication numbers aren’t the only benefits of SBIR support. An I-Corps grant from the National Institute of Neurological Disorders and Stroke provided professional development training for Kevin Harlan, a Montana Molecular early-career scientist interested in technology transfer. Harlan recently started his own biotechnology consulting company in Montana.
The neat thing about [Kevin’s] story is the amplification effect of the SBIR—how you can fund a company, [which] gives rise to others because there are opportunities for young people to learn.







