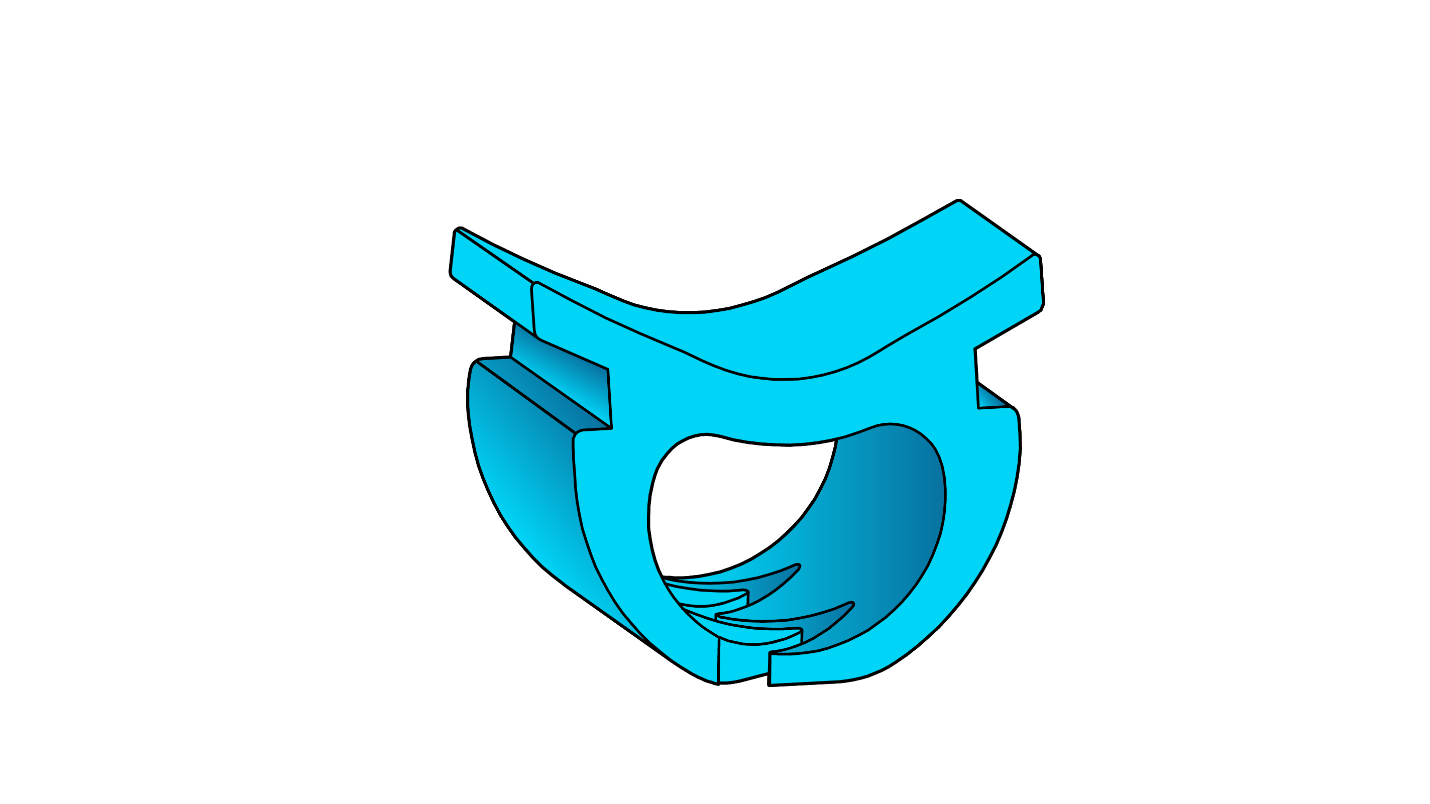
 Incidental tears in the dura mater, a tough membrane that houses cerebrospinal fluid and protects the brain and spinal cord, are one of the most common complications in lower back surgery. These tears, or durotomies, which occur in 3–14% of spine surgeries, can increase pain, risk of surgical site infection, and total hospital costs for patients. If the dura mater is torn and not properly sealed after surgery, it can also cause spinal fluid leakage, which can lead to headaches, wound infection, meningitis, revision surgery, and other severe consequences.
Incidental tears in the dura mater, a tough membrane that houses cerebrospinal fluid and protects the brain and spinal cord, are one of the most common complications in lower back surgery. These tears, or durotomies, which occur in 3–14% of spine surgeries, can increase pain, risk of surgical site infection, and total hospital costs for patients. If the dura mater is torn and not properly sealed after surgery, it can also cause spinal fluid leakage, which can lead to headaches, wound infection, meningitis, revision surgery, and other severe consequences.
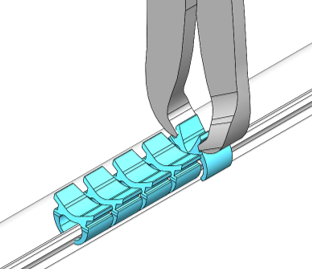
Historically, surgeons sutured dura mater to create a watertight seal, but this process can be very challenging and time-consuming, especially when minimally invasive techniques are used. To overcome these challenges, NeuraMedica, a neurosurgery device company based in Oregon City, Oregon, developed a novel surgical clip that can rapidly close the dura mater. “These clips eliminate any leakage caused by suture holes and can be used in procedures with minimal accessibility without using dural sealants,” says Rachel Dreilinger, co-founder and CEO of NeuraMedica. The company’s novel DuraFuse™ Dural Clips are non-penetrating, bioabsorbable, and imaging compatible, so they dissolve in the body over time and do not cause any imaging artifacts.
In July 2022, NeuraMedica received clearance from the U.S. Food and Drug Administration (FDA) to market and sell their DuraFuse™ Dural Clip and Applier system throughout the United States. “This is just a tremendous milestone for our company,” says Dreilinger. “It is the culmination of years of fundraising, development, testing, market research, and grant writing. We are very excited to bring a product to market that has the ability to improve patient care, reduce hospital costs, and save surgeons’ time in the operating room.”
NeuraMedica, which was founded in June 2014 when Dreilinger, a biomedical engineer, and neurosurgeon Neil Roundy M.D., secured several investment and grant funds to support their research and commercialization efforts. Their largest grant to date was a Small Business Innovation Research (SBIR) Phase II grant from the National Institutes of Health’s National Institute of Neurological Disorders and Stroke, which was awarded in 2018. “Raising money has been one of the hardest parts of the commercialization process often causing long development delays,” says Dreilinger. “The support of SBIR funding was absolutely instrumental in bringing this product to market.”
The support of SBIR funding was absolutely instrumental in bringing this product to market.
Although she has been in the biomedical engineering field for over 24 years, Dreilinger couldn’t always picture herself as an entrepreneur. “It can be challenging to be a Native woman in male-dominated fields like engineering and business,” she says. “I never imagined I would be CEO of a neurosurgery device company, yet I am here because of help and support from mentors and people who believed in me.”
Now, as CEO of a Native- and woman-owned company, Dreilinger hopes she can be an example of what is possible for other early-career scientists and engineers. “I definitely want to see more Natives, people of color, and women in engineering, business, and positions of power, so I will do everything I can in my role to support that. We have been lucky enough to have two Diné (Navajo) interns at NeuraMedica and I’m always interested in meeting other Native engineers, doctors, and entrepreneurs.”







