 As a graduate student studying biomedical engineering at the University of Virginia, Will Mauldin came across an interesting research study. It showed that poorly placed epidurals during labor, while relatively rare, still affect thousands of women during their labor experiences each year.
As a graduate student studying biomedical engineering at the University of Virginia, Will Mauldin came across an interesting research study. It showed that poorly placed epidurals during labor, while relatively rare, still affect thousands of women during their labor experiences each year.
Mauldin thought epidural failures could be avoided by building a device to help anesthesiologists guide their needle placement. He knew there was a gap in the market for ultrasound-guided solutions, even though current research showed it could improve epidural success rates. So, using his expertise in medical ultrasound technology and a deer spine from a recent hunting trip as a prototype, he partnered with fellow graduate student Kevin Owen to design an ultrasound-based spinal navigation device for placing epidurals. In 2010, Mauldin and Owen founded RIVANNA to bring their device to market.
But in 2012, the company was nearing the end of its university funding window. That same year they were awarded a Phase I Small Business Innovative Research (SBIR) grant from the National Institute of Biomedical Imaging and Bioengineering (NIBIB). “Without the SBIR funding, nobody was going to push the project any further,” says Mauldin. “That's what really allowed us to move outside of the university into our own space and hire our first employee.” The grant also attracted investors during RIVANNA’s first round of seed funding, which helped pay for technology development, patent processing, and licensing fees.
Without the SBIR program, there would not be an alternative on the market to a blind—or without image guidance—approach to performing labor epidurals for patients
In 2014, RIVANNA leaders participated in the NIH Concept to Clinic: Commercializing Innovation (C3i) Program. The entrepreneurial bootcamp helped them successfully launch their ultrasound-guided epidural technology, Accuro, which received FDA clearance in 2015.
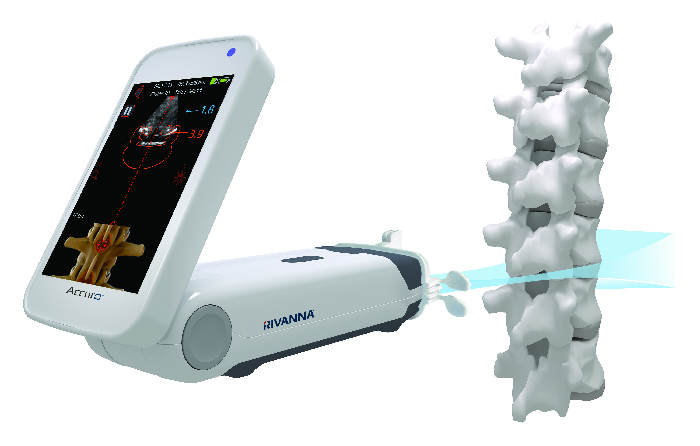 To date, the company has received more than five million dollars from five NIH institutes to develop ultrasound-based products. Currently, RIVANNA is developing ultrasound technology to improve bone marrow biopsy success rates using a Direct to Phase II SBIR grant from the National Cancer Institute (NCI). The company is already testing a prototype of the device with hopes to bring a product to market as early as 2022.
To date, the company has received more than five million dollars from five NIH institutes to develop ultrasound-based products. Currently, RIVANNA is developing ultrasound technology to improve bone marrow biopsy success rates using a Direct to Phase II SBIR grant from the National Cancer Institute (NCI). The company is already testing a prototype of the device with hopes to bring a product to market as early as 2022.
“I think the relationship with NIBIB, in particular, has been super helpful in translating this technology into the market,” says Mauldin. RIVANNA has distributed more than a thousand Accuro devices to customers in more than 20 countries. “Without the SBIR program, there would not be an alternative on the market to a blind—or without image guidance—approach to performing labor epidurals for patients,” says Mauldin.







