One of the strongest barriers to consistent physical activity in adults with type
1 diabetes is the fear that their blood sugar levels will drop too low. Arkansasbased medical device company SFC Fluidics is trying to give those people an
alternative. Company management realized their work could advance diabetic devices
by improving the technology of current pumps, one of the three components
(alongside a continuous glucose monitor and a dosing algorithm) needed for an
artificial pancreas. To improve artificial pancreas technology, SFC Fluidics set
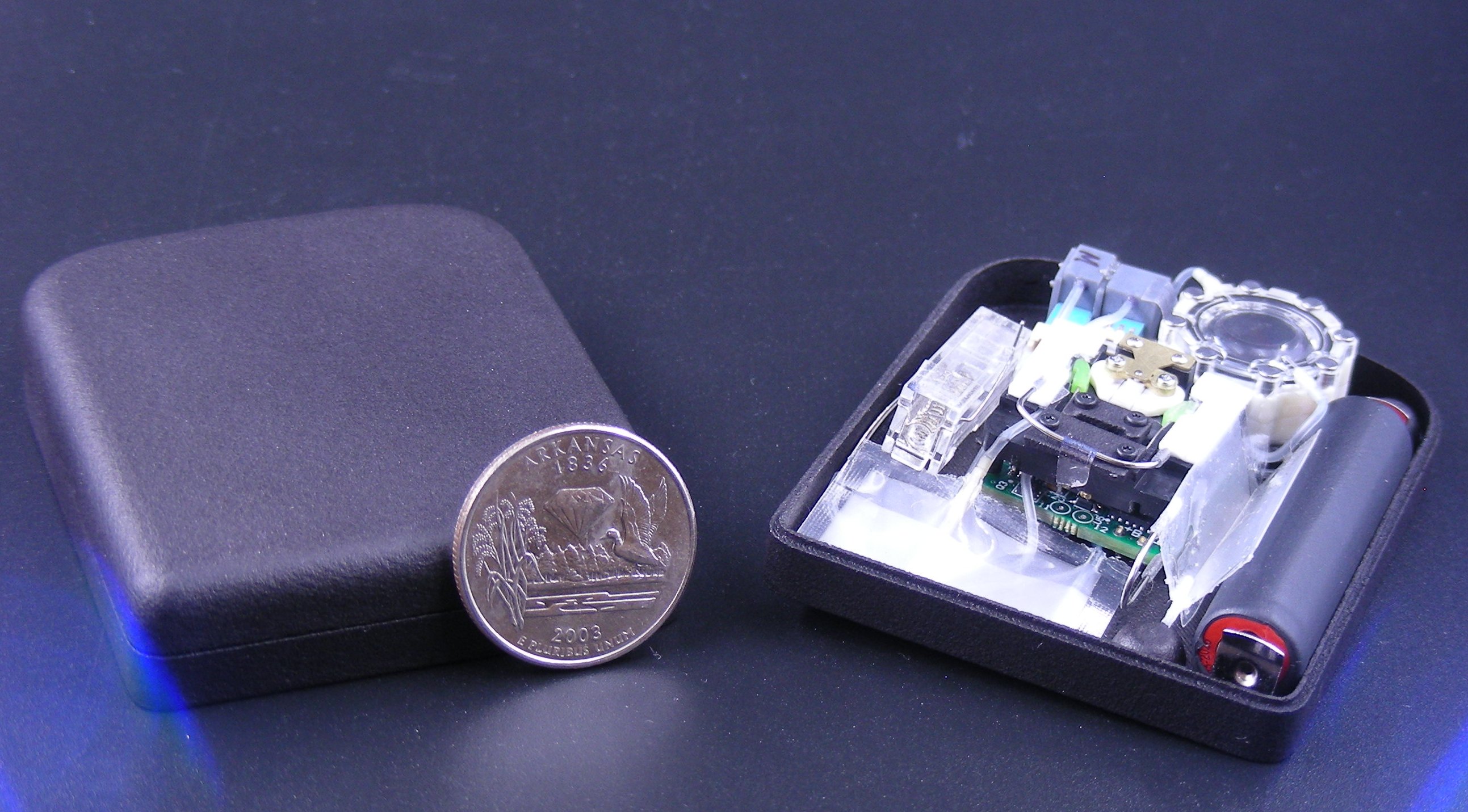
out to develop a single pump that could accurately deliver small doses of both
insulin and glucagon—usually injected by two bulky, separate pumps. To fund the development of a prototype, the company applied for and received
both a Phase I and a Phase II Small Business Innovative Research (SBIR) grant
from the National Institute of Diabetes and Digestive and Kidney Diseases.
SBIR funding also allowed the company to conduct preliminary testing required
for FDA approval. The pump they’ve designed doesn’t require mechanical parts
so it can be manufactured in smaller sizes than most other pumps, and they have
developed a sensor that is flow-based instead of pressure-based, meaning that
it can respond to blockages quicker than other systems.
SBIR funding also allowed the company to conduct
preliminary testing required for FDA approval. The funding, as well as participation in NIH’s Concept to Clinic:
Commercializing Innovation (C3i) and I-Corps, entrepreneurship programs
furthered the company’s mission. “It really puts us in a good position to go out
and start raising money,” says Payne, who adds that without SBIR-funded data,
“there would be no other way” to attract outside dollars. SBIR funding also contributed to a partnership between SFC Fluidics and the Juvenile Diabetes
Research Foundation to develop a pump that can function with any brand of
artificial pancreas. Additional SBIR grants awarded to the company have funded
the development of tether-free animal sensors that enable researchers to study
drug addiction. Payne says being an SBIR grant recipient indicates the technology is credible
and viable. But perhaps more importantly, the SBIR program is an essential
source of funding for start up biotechnology companies, “especially in those
early stages when you’ve got an idea that’s incredibly high risk. It’s very difficult
to get funding for a kernel of an idea,” says Payne.
The company is developing a new type of implanted pump for artificial
pancreases that can responsively deliver both insulin and the blood sugar-raising hormone glucagon. “Ideally, blood sugar can be corrected automatically
without you even knowing about it,” says Forrest Payne, project lead of drug
discovery at SFC Fluidics. Payne joined the company shortly after it was
founded as a spin off from its microfluidics parent company in 2003.







