 Severe burns can be life-threatening. They often require surgery to remove the damaged skin as well as the surgical harvesting of healthy skin from an uninjured site on the patient and transplantation of the skin graft to the burn injury, creating a donor site wound and leaving the patient with more wounded areas requiring care. “While autografting is effective in patients with deep partial-thickness burns, it can lead to complications, including pain and increased risk of infection and scarring,” says Allen Comer, the Senior Director, Research, Strategy and Innovation at Stratatech Corporation, a Mallinckrodt company. “To address this, we developed StrataGraft® (which consists of allogeneic cultured keratinocytes and dermal fibroblasts in murine collagen) to eliminate or reduce the need for autografting.”
Severe burns can be life-threatening. They often require surgery to remove the damaged skin as well as the surgical harvesting of healthy skin from an uninjured site on the patient and transplantation of the skin graft to the burn injury, creating a donor site wound and leaving the patient with more wounded areas requiring care. “While autografting is effective in patients with deep partial-thickness burns, it can lead to complications, including pain and increased risk of infection and scarring,” says Allen Comer, the Senior Director, Research, Strategy and Innovation at Stratatech Corporation, a Mallinckrodt company. “To address this, we developed StrataGraft® (which consists of allogeneic cultured keratinocytes and dermal fibroblasts in murine collagen) to eliminate or reduce the need for autografting.”
StrataGraft was approved by the FDA in June 2021 for the treatment of adults with thermal burns that have some remaining deep skin layers but require surgery to repair (called deep partial-thickness burns). The product is made of cells the body naturally produces that can be used in place of autograft to support the body’s own ability to heal.
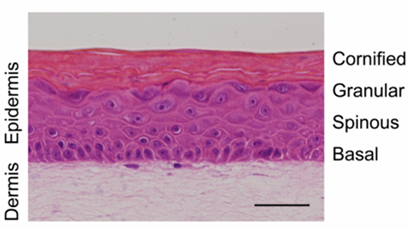
The technology underlying StrataGraft is based on research from Dr. Lynn Allen-Hoffmann’s lab at the University of Wisconsin-Madison. She and her team discovered a line of keratinocyte cells, the human skin cells that migrate across a wound to close it. The cell line was especially long-living in a culture environment, serving as a consistent source of keratinocytes for the manufacture of StrataGraft.
StrataGraft®, an FDA-approved skin tissue substitute made from cells produced naturally by the body, can be used in place of a skin graft to encourage wound healing.
StrataGraft is similar to a rectangular sheet of skin that a surgeon would harvest from a donor site. It is “meshed,” or dotted with slits, like a skin graft is when prepared for surgical use. “That was our goal,” Comer says, “to develop a product that would fit naturally within a burn surgeon’s practice.”
The FDA granted StrataGraft orphan drug designation, and it was among the first products designated by the FDA as a Regenerative Medicine Advanced Therapy (RMAT). The FDA approval is supported by data from two clinical trials of a single application of StrataGraft in patients with deep partial-thickness thermal burns. The studies were conducted at U.S. burn centers.
 “Small Business Innovation Research, or SBIR, grants from the NIH,” specifically from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of General Medical Sciences, “were critical in allowing us to get the technology for StrataGraft started,” says Comer. Stratatech has also received SBIR grants to develop more specialized skin constructs: funding from National Institute of Diabetes and Digestive and Kidney Diseases supported the development of a product for diabetic ulcers, and the National Institute of Allergy and Infectious Diseases funded a grant to develop a product for skin cancers. The late-stage development of StrataGraft was also supported with funding from the Biomedical Advanced Research and Development Authority (BARDA).
“Small Business Innovation Research, or SBIR, grants from the NIH,” specifically from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of General Medical Sciences, “were critical in allowing us to get the technology for StrataGraft started,” says Comer. Stratatech has also received SBIR grants to develop more specialized skin constructs: funding from National Institute of Diabetes and Digestive and Kidney Diseases supported the development of a product for diabetic ulcers, and the National Institute of Allergy and Infectious Diseases funded a grant to develop a product for skin cancers. The late-stage development of StrataGraft was also supported with funding from the Biomedical Advanced Research and Development Authority (BARDA).
“Stratatech is really tremendous evidence of public-private partnership to advance science,” says Derek Naten, the Executive Director of Government Affairs and Advocacy at Mallinckrodt.







