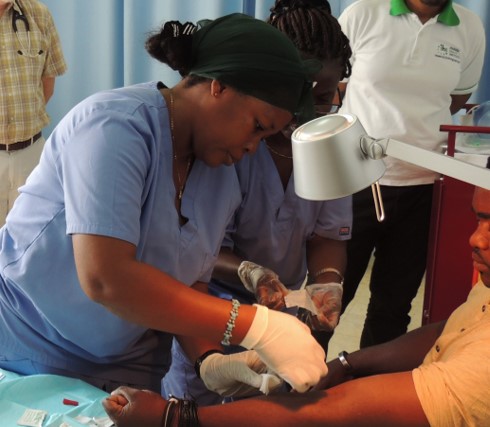
 Travelers, military personnel, and residents in malaria-stricken countries face a choice: follow a sometimes daily medication regimen that can be costly for the very poor or risk contracting a disease that sickened 219 million and killed almost half of a million people in 2017.
Travelers, military personnel, and residents in malaria-stricken countries face a choice: follow a sometimes daily medication regimen that can be costly for the very poor or risk contracting a disease that sickened 219 million and killed almost half of a million people in 2017.
While nations have tried to eradicate malaria in an effort led by the World Health Organization, early attempts largely failed and most countries now aim to reduce illnesses and deaths caused by the disease.
But new biotechnology developed by the NIH-funded company Sanaria might prove to not only protect people against malaria, but someday eradicate the disease for good.
Sanaria was founded in 2003 by Steve Hoffman to investigate a malaria vaccine after he received a Phase I Small Business Innovation Research (SBIR) grant funded by the National Institute of Allergy and Infectious Diseases (NIAID), which allowed the company to move into a small lab space.
These early funds were essential in developing their unique type of malaria vaccine
Over the next decade Sanaria was awarded two more Phase I SBIR grants, which were successfully translated into Phase II funding. Peter Billingsley, Vice President of International Projects and Strategy for Sanaria, says these early funds were essential in developing their unique type of malaria vaccine.
Many in-development malaria vaccines contain a piece of the parasite that causes malaria, but Sanaria’s approach uses intact parasites, weakened to prevent illness, and dissected directly from infected mosquitoes. Researchers had to first find the best way to extract parasites from mosquitoes without introducing contamination, and then determine the best preservation techniques. A weakened parasite won’t cause illness, but it needs to be alive to confer immunity. Billingley says the Phase II SBIR was instrumental in helping the company develop these innovative purification and preservation techniques.
NIH funding has also assisted in Sanaria’s scale up efforts, a critical step in manufacturing a commercial product.
It is this solid research, development, and manufacturing foundation that Billingsly attributes to securing funding for clinical trials, which have largely been successful. Sanaria has conducted more than 30 clinical trials in the U.S., Europe, and in seven African countries. Five of these trials showed 100 percent effectiveness against malaria infection.
 Billingsley says Phase III trials are planned for 2020 in the U.S., Germany, and West Africa, with hopes that the licensing process will begin with the Federal Drug Administration (FDA) and the European Medicines Agency (EMA) in 2021. The vaccine could be available as early as 2022.
Billingsley says Phase III trials are planned for 2020 in the U.S., Germany, and West Africa, with hopes that the licensing process will begin with the Federal Drug Administration (FDA) and the European Medicines Agency (EMA) in 2021. The vaccine could be available as early as 2022.
Immunizing travelers visiting malaria-dense areas could prevent thousands of hospitalizations in the U.S. and Europe alone. The larger impact, however, will be preventing the hundreds of millions of illnesses that occur in Africa each year, mostly in children under the age of five.
And using immunization instead of disease treatment to combat this disease opens up the door to the possibility that one day malaria will be eliminated and then eradicated from the world.







