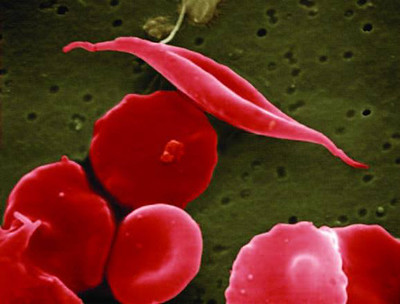
 Faulty DNA is behind many common, devastating diseases. Directly editing this DNA in humans is not yet possible, but Ohio-based pharmaceutical company EpiDestiny found a way to treat patients without editing their genetic code. Instead, they their treatment harnesses epigenetics - how organisms change which genes are turned off or on – to allow diseased cells to heal themselves. Human DNA doesn’t float freely in a cell; it is compactly wrapped up in a way that blocks access to some genes. This is how someone with brown eyes can carry the gene for blue eyes – the gene for blue eyes exists in their DNA, but in an inaccessible section.
Faulty DNA is behind many common, devastating diseases. Directly editing this DNA in humans is not yet possible, but Ohio-based pharmaceutical company EpiDestiny found a way to treat patients without editing their genetic code. Instead, they their treatment harnesses epigenetics - how organisms change which genes are turned off or on – to allow diseased cells to heal themselves. Human DNA doesn’t float freely in a cell; it is compactly wrapped up in a way that blocks access to some genes. This is how someone with brown eyes can carry the gene for blue eyes – the gene for blue eyes exists in their DNA, but in an inaccessible section.
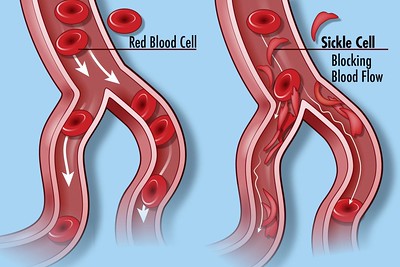
In sickle cell disease, for example, abnormal hemoglobin genes create defective cells that clog the body’s blood vessels in ways that damage organs, cause pain, and sometimes even death. But these patients still have normal hemoglobin genes: they’ve just been turned off. The EpiDestiny treatment turns these genes back on.
“The machinery in those cells is poised to turn on the healthy genes, it just lacks a key piece,” says Dr. Yogen Saunthararajah, who founded EpiDestiny in 2016. “Our drugs basically provide that piece.”
The SBIR program is a vital bridge between the world of academic research and the commercial marketplace
In April 2018, Danish pharmaceutical company Novo Nordisk acquired an exclusive license to the treatment in a deal valued at $400 million.
Since 2008, this effort has been driven forward by numerous Small Business Innovation Research (SBIR) grants from the National Heart, Lung, and Blood Institute. Saunthararajah credits the SBIR program with EpiDestiny’s success in finding a larger corporate partner. Drug development is “an incredibly expensive enterprise” ultimately requiring funds from the private sector, he says, but that funding can only come once the drug has proven itself in a clinical trial. These trials can easily cost millions.
 “It’s a catch-22,” says Sauntararajah. “And the SBIR grant was absolutely critical because it helped us cross this valley of death between the rigorous science research and all the tedious development work you need to do to get it into the clinic. SBIR enables you to get funded for that unsexy, prosaic development work that is absolutely essential if you are going to take unglamorous science into practical impact for humans.”
“It’s a catch-22,” says Sauntararajah. “And the SBIR grant was absolutely critical because it helped us cross this valley of death between the rigorous science research and all the tedious development work you need to do to get it into the clinic. SBIR enables you to get funded for that unsexy, prosaic development work that is absolutely essential if you are going to take unglamorous science into practical impact for humans.”
“The SBIR program is a vital bridge between the world of academic research and the commercial marketplace,” he says, “but it’s a whole education that scientists and doctors need to engage in so they can operate in both of these worlds successfully.







